Blog What Is Conductivity in Metals?
By: Dave Olsen
Conductivity in metal is a measure of a metal material’s ability to transmit heat, or electricity (or sound). The reciprocal of conductivity is resistance, or the ability to reduce the flow of those.
Understanding a material’s tendency to conduct may be a critical factor in the selection of that material for a given application. Clearly, some materials are chosen because they readily conduct electricity (wire) or heat (fins or tubes in a radiator). For other applications (like insulation), materials are selected because they specifically do not conduct very well.
Pure metals will tend to provide the best conductivity. In most metals, the existence of impurities restricts the flow of electrons. Compared to pure metals, then, elements that are added as alloying agents could be considered “impurities”. Alloys tend to offer less electrical conductivity than pure metals. If different properties provided by alloying are required (additional hardness or strength) it is important to choose metal materials electricity can pass through.
What Makes Something Conductive
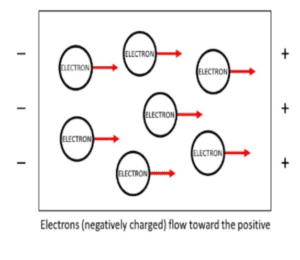
Metals conduct electricity by allowing free electrons to move between the atoms. These electrons are not associated with a single atom or covalent bond. Why do metallic bonds conduct electricity? Like charges repel each other. The movement of one free electron within the lattice dislodges those in the next atom, and the process repeats. Continually moving in the direction of the current, toward the positively charged end. This process makes metals good conductors of electricity. There are many materials electricity can pass through. MetalTek experts can help navigate creating the right alloy to meet these types of needs.
Thermal Conductivity
Thermal conductivity is like electrical in that exciting atoms in one section work to excite and vibrate adjacent atoms. That motion or kinetic energy – not unlike rubbing your hands together to get warm – allows heat to move through the metal. Alloys, which are a combination of different metallic elements, tend to offer a lower level of thermal conductivity than pure metals. Atoms of different sizes or atomic weights will vibrate at different rates, which changes the pattern of thermal conductivity. If there is less energy transfer between atoms, there is less thermal conductivity.
Pure silver and copper provide the highest thermal conductivity. Aluminum and stainless steel provide low thermal conductivity. Some materials, including copper, will readily conduct both heat and electricity. Copper is an excellent heat conductor. While others, like glass, conduct heat but not electricity.
As we have noted before, the selection of the metal for any application probably involves tradeoffs. For example, consider the choice of metal in cookware. While aluminum is a decent conductor of heat, copper conducts better and would provide quicker and more even cooking performance – if you are looking for that quick meal. But copper is much more expensive. That is why all but the highest-end cookware is made of aluminum. Aluminum with a coating or cladding is also common as aluminum is reactive to salty and acidic foods. Copper with a stainless-steel cladding would be yet another choice.
As with most of these applications, your neighborhood metallurgist, MetalTek experts, can help make a cost-effective decision on alloy selection – for conductivity or almost any other desired performance.



